Low Temperature & Ultra-low Battery
----Guoan Energy Technology (Dongguan) Co., Ltd. R&D Center, Compiled by
Definition of Low-Temperature & Ultra-Low-Temperature Batteries:
Low-temperature & ultra-low-temperature batteries are special batteries specifically developed to address the performance deficiencies of chemical power sources in low-temperature environments, capable of operating normally at extremely low temperatures (e.g., -40℃ or even lower).
 |
 |
Low-temperature -50℃ to -60℃ discharge performance at different rates (discharge capacity at -50 degrees maintains over 65% of initial capacity)
1. Definition and Characteristics
Low-temperature batteries are designed to overcome the problem of performance degradation of traditional chemical batteries in low-temperature environments. They use special materials and processes, such as the use of VGCF (vapor-grown carbon fiber) and activated carbon with a specific surface area of (2000±500)㎡/g as an additive, and inject electrolyte containing special additives to ensure stable discharge at low temperatures.
2. Application Fields
Low-temperature batteries are widely used in the following fields:
Military and Aerospace: Used for military equipment, spacecraft power supplies, etc.
Vehicle-mounted Equipment: Provides stable power for vehicles in cold regions.
Scientific Expedition and Emergency Rescue: Supports power supply for equipment in extreme environments such as polar scientific expeditions and cold region emergency rescue.
Power and Communication: Used for power grid monitoring, communication equipment, etc.
Medical Electronics: Provides power for medical equipment in low-temperature environments.
Railways, Ships, Robots: Supports the normal operation of these fields in low-temperature environments.
3. Classification
Low-temperature batteries can be classified according to performance and application fields:
By Discharge Performance: Energy storage type low-temperature lithium batteries, high-rate type low-temperature lithium batteries.
By Application Field: Military low-temperature lithium batteries, industrial low-temperature lithium batteries.
By Usage Environment: Civil low-temperature batteries (-20℃), special low-temperature batteries (-40℃), extreme environment low-temperature batteries (-50℃ and below).
4. Advantages
Low-temperature batteries have the following advantages:
Excellent low-temperature performance: Can still operate normally at -50℃ or even lower temperatures, while ordinary batteries may not be usable at -20℃.
Lightweight, high specific energy: Suitable for mobile electronic devices and portable equipment.
Long lifespan: Can maintain a longer service life even in extreme environments.
5. Background of Achievement
As the application range of lithium-ion batteries (LIBs) continues to expand, challenges are increasing, especially when operating conditions deviate from room temperature, facing more severe challenges. Although researchers have extensively studied the high-temperature performance and failure of LIBs, less attention has been paid to their performance below 0℃. The capacity loss of LIBs at low temperatures is partly due to changes in the properties of the electrolyte inside the battery. If the low-temperature performance problem of batteries can be solved, there will be no need to worry about battery usage in extremely cold places such as the polar regions and space, which will undoubtedly aid human exploration and contribute to social progress.
High energy density Li||LiCoO2 battery systems have attracted widespread attention. To apply them in extremely low-temperature conditions, continuous efforts have been made to improve the low-temperature performance of electrolytes, including liquefied gas electrolytes, new cosolvents, diluent additives, and highly fluorinated solvents. However, the improvements achieved so far remain unsatisfactory, especially regarding the electrolyte/electrode interface. Professor He Weidong's team from Harbin Institute of Technology (HIT) proposed a fluoride-sulfur electrolyte system based on lithium difluoro(oxalato)borate (LiDFOB), fluoroethylene carbonate (FEC), and dimethyl sulfite (DMS), along with isobutyl formate (IF) as an antifreeze agent, to achieve low solvation and high Li+ saturated concentration electrolyte. This electrolyte exhibits high desolvation energy, enabling efficient and reversible Li+ transport, thereby forming stable LiF-rich SEI and CEI interfaces with high Li+ conductivity and large diffusion coefficients.
This achievement was published on November 11, 2022, in a paper titled “Reconstruction of LiF-rich interphases through an anti-freezing electrolyte for ultralow-temperature LiCoO2 batteries” in Energy & Environmental Sciences.
6. Key Innovations
(1) Using isobutyl formate (IF) as an antifreeze agent, a fluorine-sulfur electrolyte was designed to achieve low coordination, high desolvation energy, and high Li+ saturation concentration electrolyte, enabling efficient and reversible Li+ transport and forming abundant F radicals to construct stable LiF-rich SEI and CEI layers with large Li+ conductivity and large diffusion coefficients;
(2) At -70℃, Li||LCO batteries using this electrolyte exhibit good cycling performance, promoting the further development of ultra-low-temperature LCO batteries.
7. Interpretation of Core Content
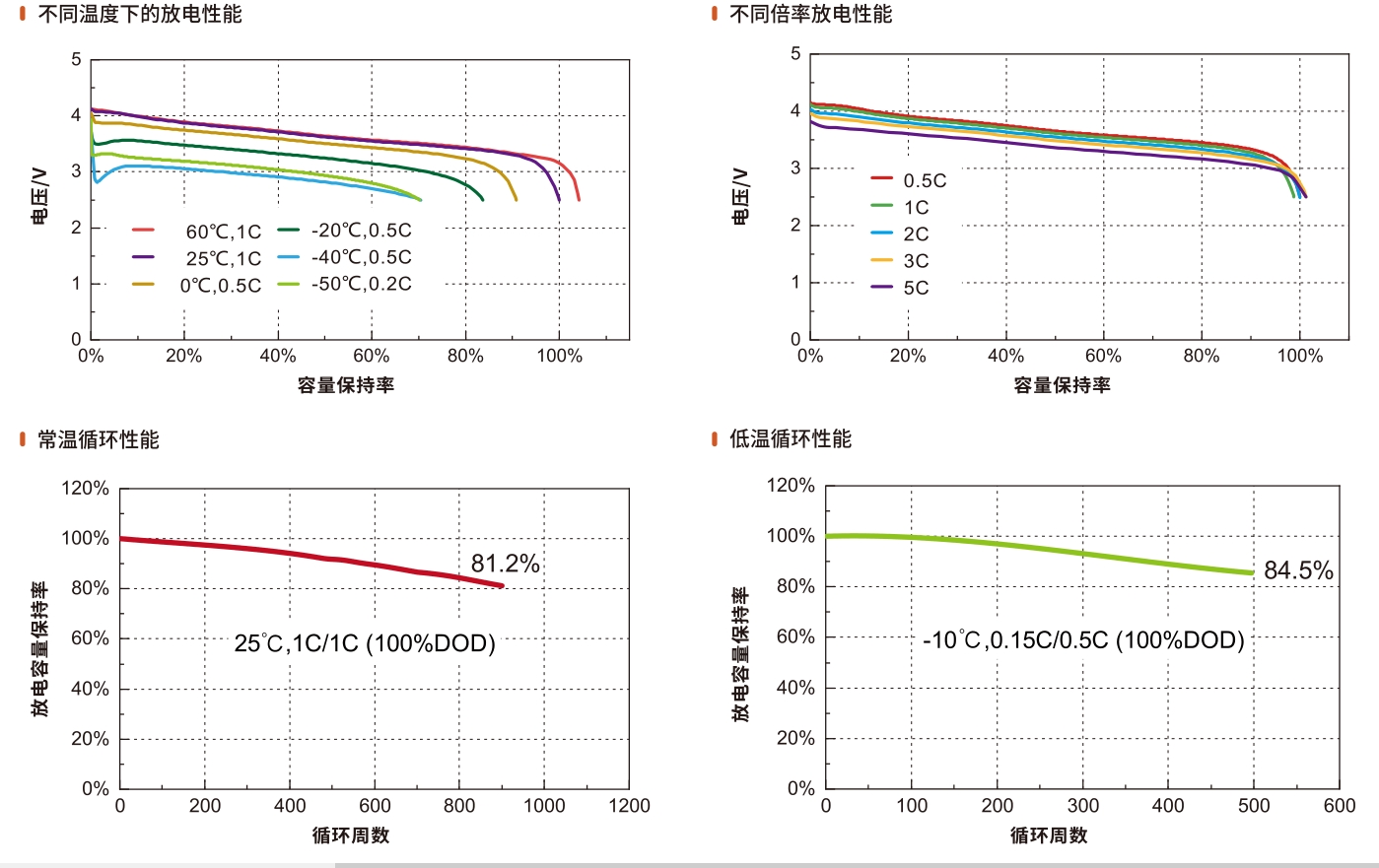
Electrolyte Design and Physical Properties: At -70°C, the schematic diagram of battery operation using electrolyte of ethylene carbonate (EC) + dimethyl carbonate (DMC) and 45% IF is shown in Figures 1a and 1d, illustrating the design concept of interfacial electrochemical performance for modified EC+DMC and 45% IF electrolytes. LiF-rich SEI and CEI layers enable efficient Li+ transfer and stable electrode/electrolyte interfaces (Figure 1d). Conversely, LiF-poor SEI and CEI layers in EC+DMC electrolyte lead to rigid Li + transfer and dendrite growth. As a diluent for 45% IF electrolyte, IF enables Li + 's small coordination number (CN), ensuring Li + efficient conduction at low temperatures. Therefore, compared to EC+DMC and FEC+DMS electrolytes, IF-based mixed electrolytes combine the advantages of IF agent and FEC+DMS electrolytes, making them more stable in low-temperature and high-voltage applications.
[Figure 1] Schematics of EC+DMC (a) and 45% IF (d) electrolytes; Li+ solvation structure and corresponding desolvation energy of EC+DMC (b) and 45% IF (e) electrolytes; migration paths determined from topological analysis of EC+DMC (c) and 45% IF (f) electrolytes; (g) Li+ saturation concentration of different electrolytes at specific temperatures; (h) ionic conductivity of different electrolytes; (i) comparison of capacity and corresponding cycle life of 45% IF electrolyte with representative low-temperature electrolytes.
Figures 1b-c and e-f show the Li+ solvation structure, corresponding desolvation energy, and migration paths determined from topological analysis. The desolvation energy of 45% IF electrolyte is much higher than that of EC+DMC electrolyte, as shown in Figures 1b and 1e. In EC+DMC electrolyte, the overall movement path of Li+ remains largely unchanged, limiting Li+ deviation, providing smaller diffusion coefficient and ionic conductivity, and the region becomes too small for Li+ migration, as shown in Figure 1c. Radial distribution function (RDF) data analysis indicates that EC+DMC electrolyte exhibits a characteristic solvent-separated ion pair (SSIP) structure, where Li+ coordination is controlled by EC, DMC, and PF6- molecules (Figure 4b). Due to the higher freezing points of EC and DMC solvents at ~39°C and ~3°C, Li+ transport is limited at -20°C. In contrast, the trajectory range of Li+ is larger, and fully connected transferable channels can be seen in Figure 1f, leading to larger diffusion coefficient and ionic conductivity.
The saturation concentration of Li+ is proposed to analyze the solvation degree of Li+, as shown in Figure 1g. At -70°C, the 45% IF electrolyte with added antifreeze IF has a low solvation degree and high Li+ saturation concentration, ensuring efficient Li+ migration. Figure 1h shows the ionic conductivity of 45% IF, FEC+DMS, and EC+DMC electrolytes. Figure 1i summarizes a detailed comparison of different low-temperature electrolytes reported in the literature, where the 45% IF electrolyte exhibits the best overall performance in terms of capacity and cycle life at -70°C.
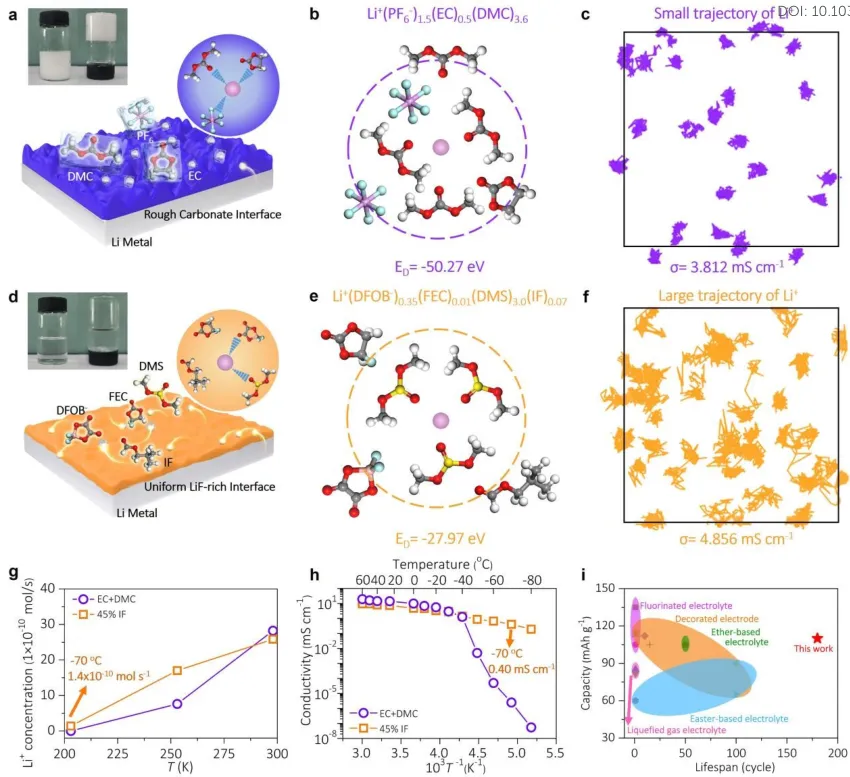
Low-temperature behavior: Li||LCO cells with assembled areal capacities of ~0.82 mAh cm-2 and ~2.74 mAh cm-2 were used to investigate anode stability under experimental and practical conditions, respectively. Figure 2a shows that compared to EC+DMC electrolyte (60°C to -20°C), 45% IF electrolyte has a wider operating temperature range, from 60°C to -70°C. Figure 2b shows that cells using 45% IF electrolyte have superior rate performance compared to the control group. As shown in Figure 2c, at 1/3 C and -20°C, Li||LCO cells using 45% IF electrolyte achieved a maximum capacity of 156 mAh g-1 and 93.5% capacity retention after 500 cycles. In Figure 2d, even at -70°C, the cell can achieve a capacity of 110 mAh g-1 after 170 cycles.
[Figure 2] Electrochemical performance of Li‖LCO cells (2.70-4.45V) using different electrolytes, with LCO loadings of ~3 mg cm-2 and ~10 mg cm-2, respectively. (a) Temperature performance from 60°C to -70°C at different discharge rates. (b) Rate performance and (c) cycling performance of cells using different electrolytes at 1/3 C at -20°C. (d) Cycling performance of cells using 45% IF electrolyte at 1/15 C and -70°C. (e) Cycling performance of cells containing EC+DMC and 45% IF electrolytes at 1/3 C and -20°C, with LCO loading of about 10 mg cm-2. (f) Cycling performance of cells using 45% IF electrolyte at 1/15 C and -70°C, with LCO loading of about 10 mg cm-2. (g) Low-temperature discharge curves. Cells were charged at different temperatures and discharged at -70°C using 45% IF electrolyte. (h) Optical image of an electric toy powered by Li‖LCO cell and 45% IF electrolyte at -70°C. (i) Comparison of current work with recent related works on low-temperature batteries.
For practical applications, high-load LCO cathodes of about 10 mg cm-2 (areal loading of about 2.74 mAh cm-2) were investigated. As shown in Figure 2e, the capacity of the cell with EC+DMC electrolyte decayed rapidly within 25 cycles. As shown in Figure 2f, cells with 45% IF electrolyte exhibited capacities of 146 mAh g-1, 124 mAh g-1, and 109.7 mAh g-1 at 1/15 C and -50°C, -60°C, and -70°C, respectively. In Figure 2g, the initial coulombic efficiency of the 45% IF electrolyte cell was 88% when charged at room temperature and discharged at -70°C, and 69% when charged and discharged at -70°C. Figure 2h shows the LCO battery using 45% IF electrolyte at -70°C.
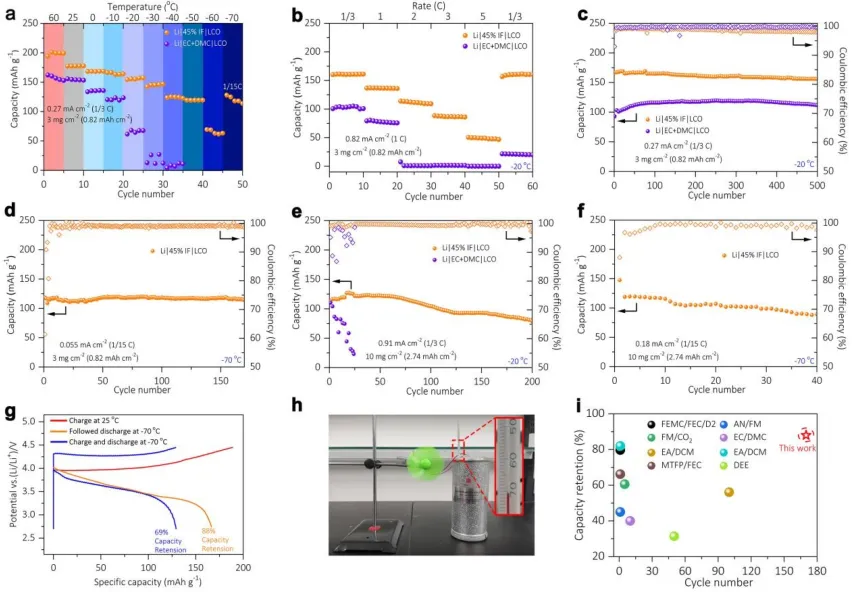
SEI and CEI layer composition: Schematics describing the deposition behavior of Li+ in EC+DMC and 45% IF electrolytes at -20°C are shown in Figures 3a and 3e. Conventional electrolytes are prone to dendrite formation below -20°C due to small nucleation size and slower Li+ diffusion compared to room temperature, leading to uneven deposition. The Li metal surface in EC+DMC electrolyte shows a large number of mossy and needle-like dendrites unevenly distributed, resulting in a porous microstructure with smaller Li+ conductivity and diffusion coefficient, as shown in Figure 3b. In contrast, the lithium metal surface with 45% IF electrolyte appears elliptical, with a dense dendrite-free and flat interfacial structure, as shown in Figure 3f.
Optical digital microscopy reflects the roughness of the lithium metal anode surface after low-temperature cycling. Figures 3d and 3h indicate relatively stable Li+ deposition and a smooth anode/electrolyte interface at -20°C. This morphology is beneficial for reducing the surface area for SEI growth and inhibiting the formation of dead lithium, thereby improving cycling performance at low temperatures.
[Figure 3] SEI characteristics obtained by SEM, optical digital microscopy, finite element simulation, and in-situ optical microscopy. Schematics describing the deposition behavior of Li+ in EC+DMC (a) and 45% IF (e) electrolytes. Top-view, cross-section, and optical digital microscopy SEM images of EC+DMC (b-d) and 45% IF (f-h) electrolytes. Finite element simulation of morphology evolution of EC+DMC (i) and 45% IF (k) electrolytes. (j, l) Cycling performance of symmetric Li‖Li cells fabricated with 45% IF and EC+DMC electrolytes at -20°C under a current density of 0.5 mA cm-2 and a fixed capacity of 0.5 mAh cm-2. In-situ optical microscopy images of lithium deposits in EC+DMC (m) and 45% IF (n) electrolytes at 30, 60, 90, and 120 minutes at a current density of 0.5 mA cm-2.
Phase-field simulations were used to numerically investigate the electric field distribution at the lithium metal/electrolyte interface, as shown in Figures 3i and 3k. As shown in Figures 3b-c, many mossy and needle-like dendrites can be seen on the lithium metal anode using EC+DMC electrolyte. However, the lithium metal anode deposited with 45% IF electrolyte shows a thick and low-tortuosity surface, as shown by phase-field simulation and cryogenic FIB. In addition, symmetric Li||Li batteries were also studied at -20℃, as shown in Figure 3j. The symmetric Li||Li battery with 45% IF electrolyte showed stable cycling for over 4000 hours, while the symmetric Li||Li battery with EC+DMC electrolyte failed after 500 hours, as shown in Figure 3l.
In addition, Li||Cu batteries were fabricated into a colorimetric cup-type device to study the cross-sectional morphology of the Cu surface with different electrolytes at -20℃ by in-situ optical microscopy. The images recorded at different times are shown in Figure 3m. Under 45% IF electrolyte at 20℃, uniform and dense Li deposition will be formed, resulting in a smooth surface without dendritic structures.
Low-solvation electrolyte and in-situ verification: The solvation structure was studied by computational simulation, Raman spectroscopy, and Fourier transform infrared spectroscopy (FT-IR). Classical molecular dynamics (MD) simulations and RDF data were also used to analyze the SSIP and CIP structures in EC+DMC and 45% IF electrolytes, respectively. Figure 4g shows the CN of Li+ between different electrolyte components. Compared with FEC and IF, Li+ is more inclined to coordinate with DMS with larger CN.
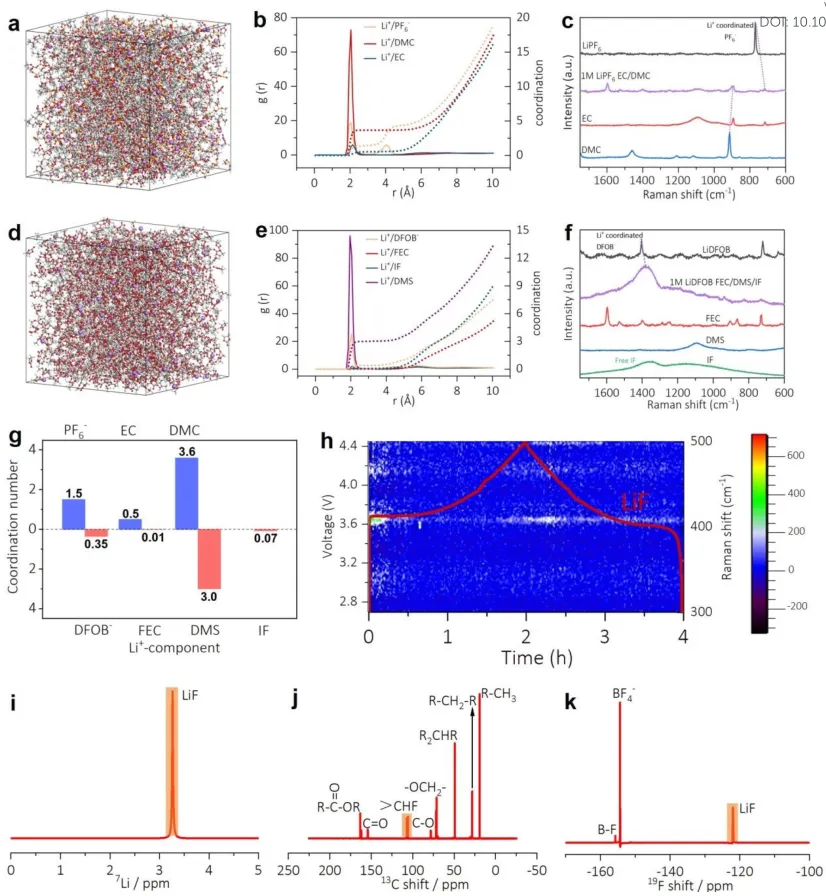
Theoretical and experimental evidence of electrolyte structure and transformation. Snapshots (a, d) and RDF (b, e) were obtained from MD simulations and Raman spectroscopy (c, f) of 45% IF (a-c) and EC+DMC (d-f) electrolytes at -20℃. (g) CNs between Li+ and different electrolyte components. (h) In-situ Raman spectroscopy of LCO batteries using 45% IF electrolyte in the first charge/discharge cycle. (i-k) 7Li, 13C, and 19F liquid-state NMR spectra of 45% IF electrolyte residue after the 50th cycle at -20℃.
Figure 4h illustrates the charge/discharge process using time-resolved Raman images from 300 cm-1-500 cm-1. As shown in Figure 4g, a large amount of LiF is formed during charging and exists in the electrolyte throughout the discharge process, which is attributed to the abundant F- from the cracked FEC and LiDFOB and the strong ionic affinity of Li+.
Nuclear magnetic resonance (NMR) was used to study the composition of the commercial electrolyte and the post-reaction 45% IF electrolyte residue, as shown in Figures 4i-4k. A significant LiF component was found in the electrolyte after cycling at -20℃, indicating that a large amount of LiF was formed in the 45% IF electrolyte during the charge-discharge reaction, forming a stable LiF-rich SEI and CEI layer. Combined with the 13C shift results of the 45% IF electrolyte, it indicates that the FEC molecule is reduced during the cycle, and the F radical combines with Li+ to form abundant LiF groups through a single-electron reaction.
8. Implications of the Results
A low-solvation, high-desolvation energy, and high Li-saturation concentration electrolyte was developed by introducing IF antifreeze agent into the LiDFOB/FEC/DMS fluorosulfur electrolyte system. + The electrolyte has excellent physical and chemical properties. This electrolyte can achieve a LiF-rich stable SEI and CEI layer, with high Li + conductivity and large diffusion coefficient, wide reversible working temperature window, and other advantages. In-situ optical microscopy combined with MD simulation and phase-field simulation shows that the new electrolyte makes Li on the lithium metal anode + deposition uniform, which helps to achieve a long-life reversible symmetric Li||Li battery (>4300 hours) at -20℃. In addition, the battery using this electrolyte has a discharge capacity as high as 110 mAh g -1 after 170 cycles at -70℃. The compatibility of the modulated electrolyte with lithium metal anodes and commercial 4.45V LCO cathodes provides a promising pathway for developing high-energy LCO batteries with a wide operating temperature range. This work represents significant progress in the development of practical low-temperature LCO batteries based on efficient electrolyte design.
----Guoan Energy Technology (Dongguan) Co., Ltd. R&D Center, Compiled by
Service Hotline
Email:zhangjie@ganeny.com
Website:www.ganeny.com
Address: No. 79, Shayuan Road, Shabu, Dalang Town, Dongguan City, Guangdong Province, China

Official Public Account
Privacy Policy | SEO | Support:300.cn
